Pharmaceutical drugs, capable of seriously impacting human life and health, represent great public interest. They are therefore subject to strict legal and regulatory control. Largely for this reason, it is extremely costly and time-consuming to develop new pharmaceutical drugs.
Types of pharmaceutical drugs
Pharmaceutical drugs can be divided into prescription drugs and over-the-counter (OTC) drugs. Prescription drugs can be further divided into originally developed “brand-name” drugs (new drugs) and generic drugs. Nippon Shinyaku manufactures brand-name drugs on the basis of its research and development (R&D).
| Pharmaceutical drugs | Prescription drugs (Drugs dispensed at a pharmacy on the presentation of a medical prescription) |
Brand-name drugs (new drugs) (Drugs that require governmental authorization to be sold upon the verification of the efficacy and safety of their novel active ingredient or ingredients) |
|---|---|---|
| Generics (generic drugs) (Drugs which contain the same active ingredients as brand-name drugs and which are manufactured and sold upon the patent expiration of the brand-name drugs) |
||
| Over-the-counter (OTC) drugs | Drugs that may be directly and readily purchased at a pharmacy or other authorized sales outlet |
Pharmaceutical market
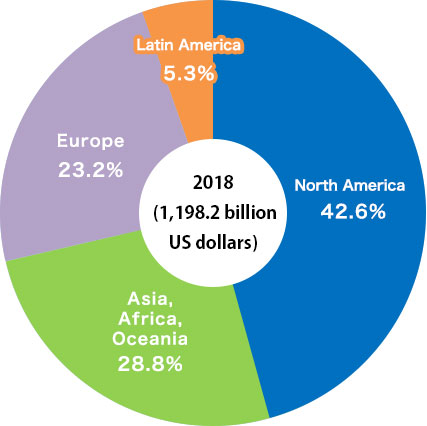
The pharmaceutical market represents about 130 trillion yen in the world and about 9 trillion yen* in Japan.
Japan is the third largest pharmaceutical market in the world after the United States and China.
(Converted at the rate of 1 US dollar = 110 yen)
* “Sales of pharmaceutical drugs in major countries” in Japan Pharmaceutical Manufacturers Association DATA BOOK 2020
Data: Ministry of Health, Labour and Welfare of Japan, “Annual Report on Statistics of Production by Pharmaceutical Industry”
Source: Japan Pharmaceutical Manufacturers Association DATA BOOK 2020
The Japanese pharmaceutical market is currently facing a challenging situation due to the governmental policy of promoting the use of generic drugs in an attempt to reverse the trend of rising social security expenses. In such a situation, Nippon Shinyaku is vigorously working on global business expansion while continuing its R&D to produce epoch-making new drugs.
For example, one of Nippon Shinyaku’s original drugs, Uptravi, treatment drug for pulmonary arterial hypertension, is being promoted worldwide by the company’s global partner Johnson & Johnson. Nippon Shinyaku has marketed on its own another original drug, Viltepso, for the treatment of Duchenne muscular dystrophy, in the United States.
Drug discovery process
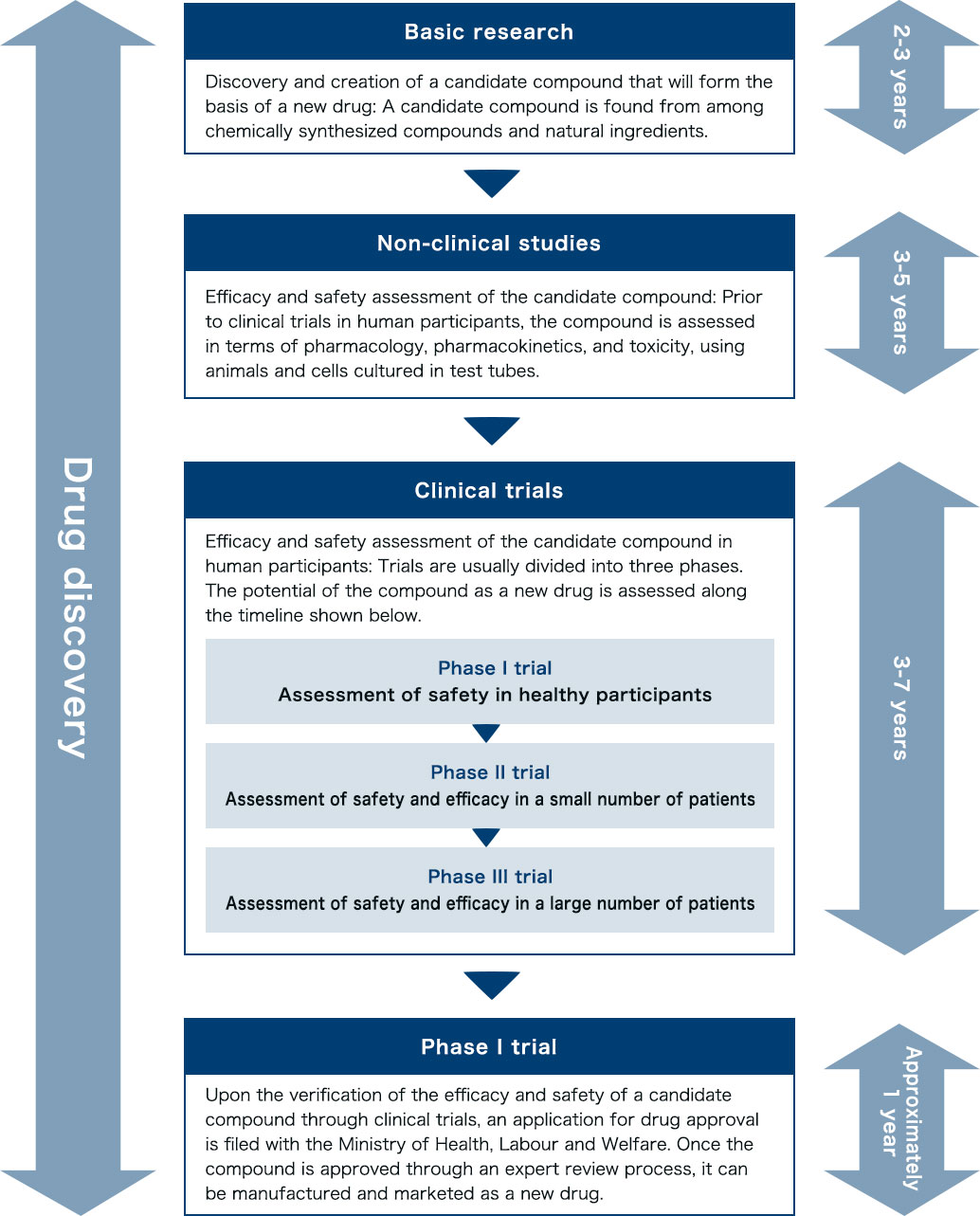
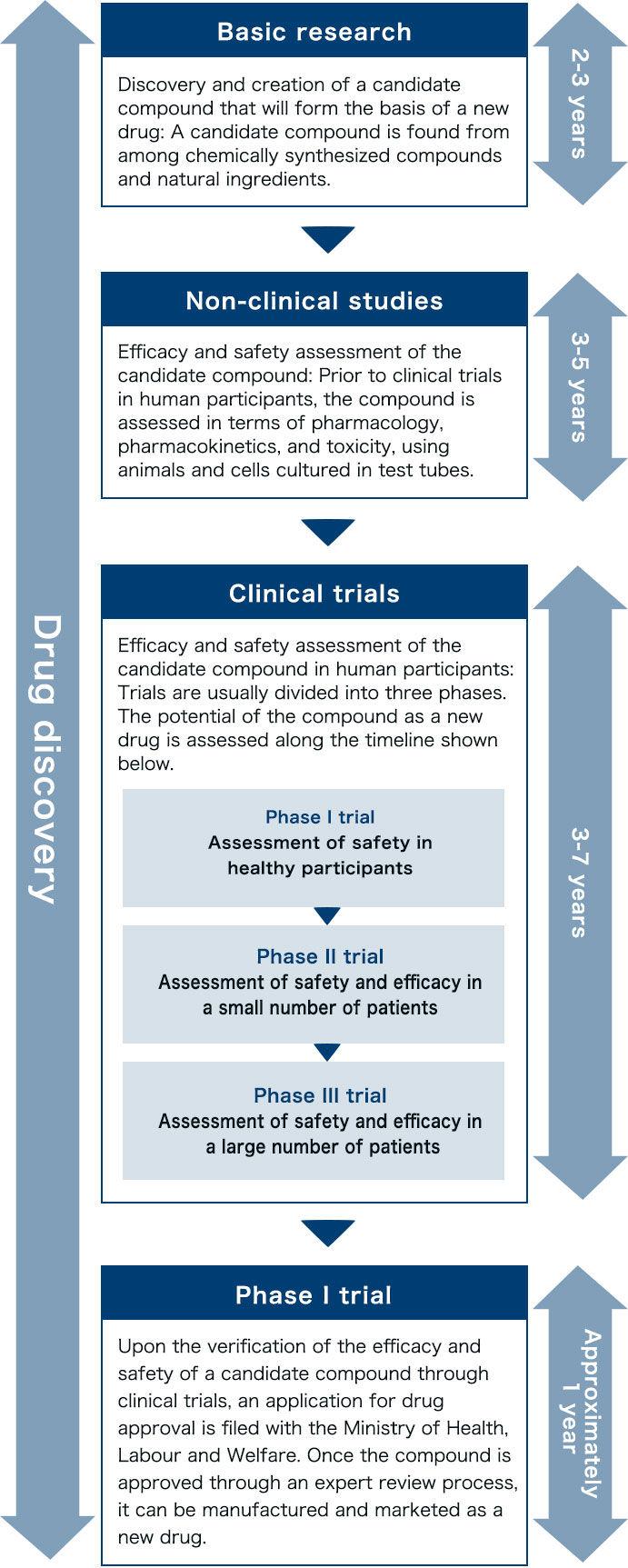
In Japan, the rate of drug discovery projects successfully resulting in a launch is extremely low at about one in every 30,000. In this highly challenging situation, most candidate compounds are abandoned in the middle of the drug discovery process.
New drug development must undergo the stages of basic research, non-clinical studies, and clinical trials. The manufacturing and marketing of a new drug are possible only after successful clinical trials and approval by the Minister of Health, Labour and Welfare. New drug development is known to be extremely time-consuming, requiring in many cases over 10 years until launching.
R&D cost
It is generally known that R&D represents a much larger cost component in the pharmaceutical industry than in other industrial sectors. Continuous investment is essential for the development of quality new drugs.
As one of the Six Actions of its 6th Five-Year Medium-term Management Plan, Nippon Shinyaku embraces the goal of “Creation of new value through R&D” and plans to invest a total of some 120 billion yen in R&D in five years.
Creating new value by adding new modalities and technologies on the company’s drug discovery foundation, which has produced NS-304 (low molecular drug) and NS-065/NCNP-01 (nucleic acid drug), thereby expanding the spectrum of drug discovery
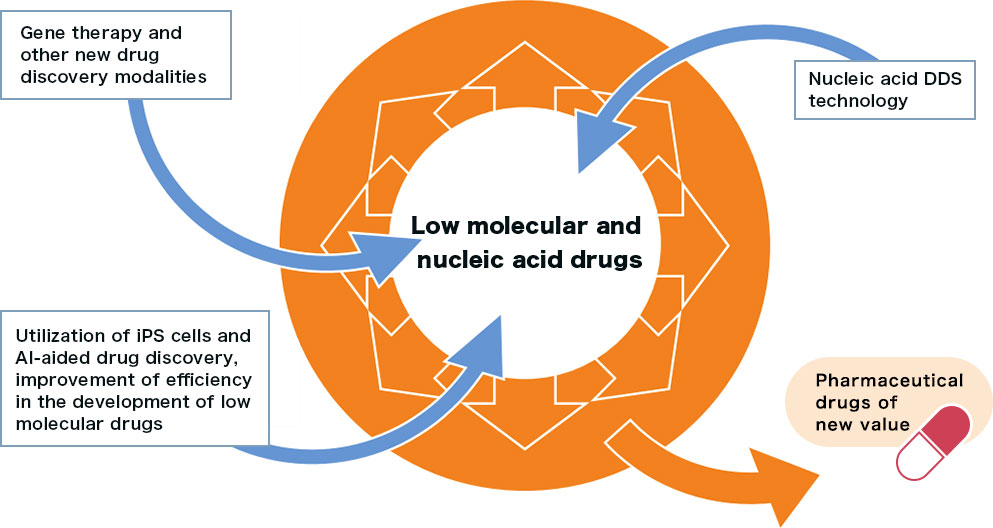
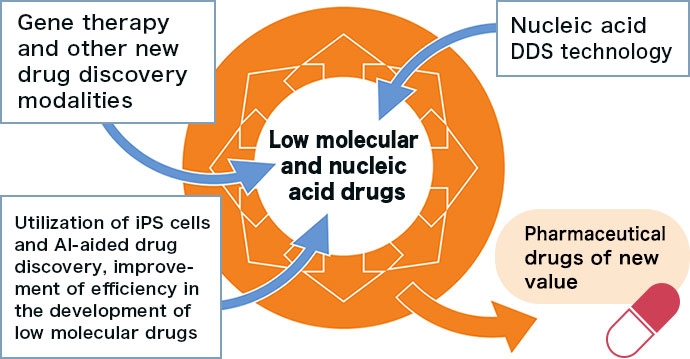
To propose innovative treatment drugs for diseases with significant unmet therapeutic needs, which have not been addressed by other pharmaceutical companies, Nippon Shinyaku continues active R&D investment, constantly strengthening its R&D pipeline along the three pillars of in-house drug discovery, in-licensing, and product life cycle management (PLCM).
To Our Individual Investors
- Message from the President
- About the Pharmaceutical Industry
- Nippon Shinyaku’s DNA
- Business/Financial Information
- Stock Information
- FAQ
- Shareholders’ Privileges







